This week’s science Sunday is geared towards an older child audience. I must confess that the furthest my 3 year-old and I got on the conversation of salt crystals was to watch the time elapsed video below and me to say, “Look! Salt Crystals are growing over time. Wow, isn’t that cool?! Just like we had on our window when we painted it…” And Holden responding, “Yeah.”
This week’s science Sunday is geared towards an older child audience. I must confess that the furthest my 3 year-old and I got on the conversation of salt crystals was to watch the time elapsed video below and me to say, “Look! Salt Crystals are growing over time. Wow, isn’t that cool?! Just like we had on our window when we painted it…” And Holden responding, “Yeah.”
Science Done.
If your kids are a bit older and/or super smart, I think they will appreciate learning more about salt crystals. For the record, snow is a crystal too, and we’ll learn more about that one in a few weeks. It’s super cool stuff. I can’t even wait.
So to start things off, a crystal is a solid material whose atoms form a repeated, ordered arrangement called a lattice. They can take on many different shapes (lots of them with fancy names!) but thankfully, salt crystals form a cubic shape.
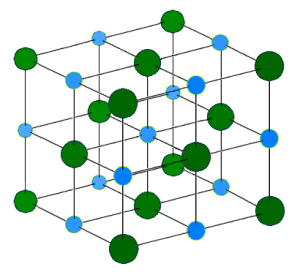
A salt crystal lattice looks like this:
Source: Wikimedia Commons
Bam! Blue is Sodium. Green is Chlorine. (When combined, they form the ionic compound, sodium chloride.) Easy peasy.
So how exactly do we go from salt water in liquid form to a solid crystal like what happened when we painted with snow?
I’m so glad you asked, because this is the fun part!
So in salt water, there are all these ions floating around — positively charged sodium ions and negatively charged chlorine ions. These ions are attracted to each other. As the water evaporates, these ions travel individually to the lattice that’s forming and attach themselves to it in an orderly fashion. In this way, the crystals grow and grow. AND, the thing that makes crystals crystals is that they ALWAYS attach to each other in the same orderly fashion. Like, the chloride ions are never going to form a coups and all line up on one side of the crystal and block the sodium ions out and form some crazy, irregular shape. It just won’t happen. It’s not what they do. If pure salt water evaporates, it’s gonna look like the pretty lattice that you see above. Period. Crazy, right?! It’s so ordered it kinda blows my mind considering how crazy and unordered this world often seems to be.
Below you can see what it looks like in elapsed time for salt crystals to form from salt water. I thought this video was pretty cool. Then I learned that an elementary school class created it, and I thought it was even cooler! Kids rule.
I hope you enjoyed Science Sunday even though I kind of flipped the geek out!