
I have really enjoyed our unit on fire so far. Some of our book review choices were very interesting, and who doesn’t like to have a backyard fire to relax at the end of the evening? So now that we have seen a fire in action, lets learn a little bit about fire. Since I am not a chemist, and the point of Science Sunday is to be able to teach our children a little something, I will try to be as basic as I can with the science of fire.
I think one of the most interesting things about fire is that it can be both extremely helpful or extremely harmful. For instance, a controlled fire for cooking, heat, or light is helpful. But an uncontrolled fire can harm individuals or their possessions very quickly. I had never really taken the time to think about things in the natural world this way, but I think this will make for a good discussion with the kids!
Fire is not matter like much of the world around us. Fire is a chemical reaction that produces heat and light. Fire is created when oxygen combined with a fuel (such as wood) is combined with enough heat to cause a chain reaction. This is referred to as the fire tetrahedron.
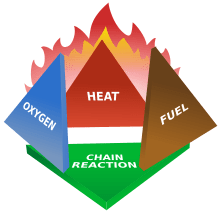
Each type of fuel has its own ignition temperature (kindling point), the lowest temperature at which the fuel will spontaneously ignite and begin the chemical reaction of fire. The heat source that heats the fuel to its ignition temperature can come from sources such as lightning, friction, or another fire. So once a fire starts, it causes a chain reaction. As the website How Stuff Works said “The dangerous thing about the chemical reactions in fire is the fact that they are self-perpetuating. The heat of the flame itself keeps the fuel at the ignition temperature, so it continues to burn as long as there is fuel and oxygen around it.”
A fire does not consume every bit of its fuel. In a wood fire, smoke is a volatile gas given off by the chemical reaction. Char is carbon left over from the fire, and ash is composed of noncombustible minerals in the wood.
I definitely learned something about fire by researching this topic. The Fire Tetrahedron may sound boring, but it sure does produce something beautiful! Come back on Tuesday when we make fire starters for your next camp fire.


